As electric vehicles (EVs) continue to gain popularity, attention is increasingly drawn to their most vital component—the battery. Of the various battery types available, lithium iron phosphate (LiFePO4) has built a strong reputation for safety and stability. Yet, like all lithium-ion technologies, it is not completely free from the risk of thermal runaway—a hazardous chain reaction that can lead to overheating, fire, or even explosion. This article examines the causes of LiFePO4 thermal runaway, its possible outcomes, and recommended strategies to reduce the associated dangers.
Battery thermal runaway happens when a cell’s internal temperature rises uncontrollably, exceeding the system’s capacity to release heat. This self-sustaining reaction can cause battery materials to break down, release gases, and ignite.
In electric vehicles, this presents a serious challenge. Lithium batteries store substantial energy in a small space. When failures occur—such as internal short circuits or overcharging—the resulting chemical reactions produce intense heat. Combined with flammable vehicle components such as seat foam and wiring insulation, fires can spread quickly.
The process of thermal runaway in LiFePO4 and other lithium-ion cells involves a sequence of interconnected chemical and physical stages:
- SEI (Solid Electrolyte Interface) Breakdown: Between 80–120 °C, the protective SEI layer decomposes, exposing the anode to direct reactions with the electrolyte.
- Separator Failure: Polyethylene and polypropylene separators begin to shrink or melt around 130–170 °C, disrupting ion flow. Above 190 °C, the separator may rupture, leading to internal short circuits.
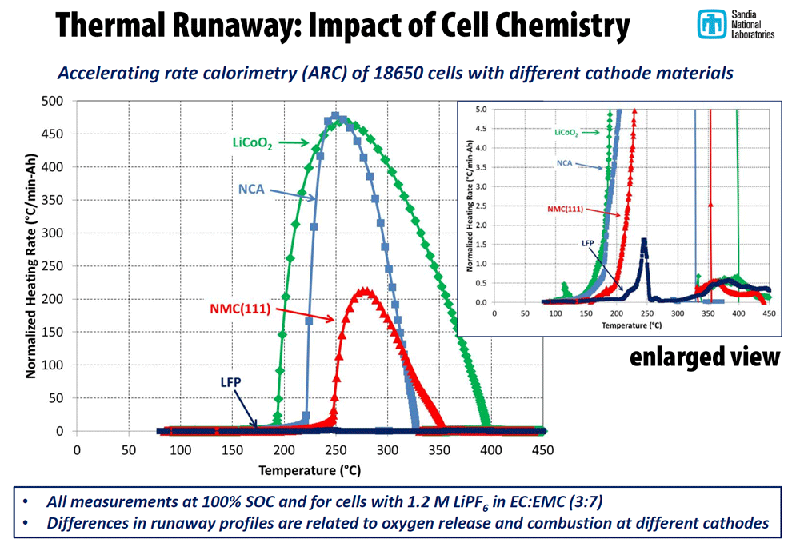
- Electrode Breakdown: Cathode materials can release oxygen, which reacts violently with the electrolyte and speeds up heat generation.
- Pressure Buildup: Rapid gas generation increases internal pressure until vents burst, possibly resulting in fire or explosion.
This chain of events explains why thermal runaway, once triggered, is so destructive and hard to contain.
While LiFePO4 batteries are known for being safer than other lithium-based chemistries due to their stable cathode structure, they are still susceptible under severe conditions. Key triggers include:
1. Overheating
High external temperatures or ineffective cooling can cause excess heat to build up inside cells.
2. Overcharging and Over-Discharging
Operating a LiFePO4 cell outside its voltage limits accelerates internal wear and raises heat output.
3. Internal Short Circuits
Defects from manufacturing, physical harm, or dendrite growth can create short circuits that release sudden heat.
4. Physical Damage
Impacts, punctures, or crushing can damage separators and electrode structures, leading to electrical failure.
5. Thermal Stress
Heat from adjacent failing cells or faulty cooling systems can start a chain reaction within the battery pack.
These abuse conditions—mechanical, electrical, and thermal—often interact, increasing the overall risk. For example, a crash (mechanical) may cause a short circuit (electrical), producing heat (thermal) that escalates into full runaway.
Although LiFePO4 is inherently more stable than nickel-based lithium chemistries, thermal runaway remains possible under certain circumstances:
- Production Defects: Inadequate quality control can introduce tiny flaws that result in internal shorts.
- Improper Use: Repeated overcharging or deep discharging strains the battery and increases thermal risk.
- Environmental Stress: Exposure to extreme heat, crushing, or penetration can weaken cell integrity.
The important difference is that LiFePO4 generally requires more severe mistreatment to enter thermal runaway compared to chemistries like NMC or LCO—but the danger is still present.
The impact of a thermal runaway event goes well beyond a single cell:
1. Loss of Battery Performance
Runaway raises cell temperature, reducing capacity, hindering charge/discharge cycles, and shortening service life.
2. Fire and Explosion Risks
High heat and oxygen release can ignite the electrolyte, causing fires or explosions that threaten occupants and property.
3. Vehicle Inoperability
In EVs, a damaged battery pack can render the vehicle unusable, requiring expensive repairs and leading to safety recalls.
Reducing the risk of thermal runaway requires a multi-layered strategy combining engineering, manufacturing quality, and user responsibility:
1. Battery Balancing Systems
Use a battery management system (BMS) to maintain even charge across cells and prevent overcharging or deep discharge.
2. Improved Cooling Systems
Implement liquid cooling or heat pipes to effectively remove heat, especially in large EV battery packs.
3. Real-Time Temperature Monitoring
Incorporate sensors and predictive software to detect early signs of overheating and isolate affected modules.
4. Stringent Safety Testing
Apply non-destructive inspection methods such as X-ray or ultrasound to identify hidden flaws before batteries are put into use.
5. Quality-Centered Manufacturing
Enforce strict production standards to reduce defects that could cause short circuits or instability.
6. User Education and Maintenance
Inform users about correct charging practices and safety measures to prevent misuse.
7. Emergency Protocols
Install fire suppression systems in EVs and establish clear response plans for thermal incidents.
8. Battery Recycling Programs
Develop safe recycling and disposal channels to avoid hazards after a battery’s useful life.
9. R&D into Safer Chemistries
Continue developing solid-state electrolytes and new cathode materials with better thermal stability.
10. Government Regulations
Stronger policies and safety standards can promote industry-wide compliance and safer EV adoption.
Thermal runaway in LiFePO4 batteries may be less frequent than in other lithium-ion types, but it remains an important safety issue. Its causes are varied—from internal flaws to external abuse—and the outcomes can be serious. Thankfully, through reliable battery management systems, thermal regulation, high-quality production, and regulatory oversight, these risks can be reduced.
As EV use grows, ongoing investment in safer battery technologies and cross-industry cooperation will be essential to building public confidence and ensuring long-term dependability.
Next:EVE Energy Shines with Full-Scenario Energy Storage Solutions at SNEC ES+ 2025
Previous:CORNEX Unveils Next-Gen “Everest” 6C Fast-Charging Battery Cell