With advancing technology, electric vehicles (EVs) have become increasingly prevalent. The battery, as the core component of an EV, plays a critical role in ensuring safety and stability. In recent years, thermal runaway in LiFePO4 (lithium iron phosphate) batteries has gained significant attention. This article explores the causes, impacts, and preventive measures related to LiFePO4 battery thermal runaway.
Battery thermal runaway refers to a critical condition in which the internal temperature of a battery rises rapidly during use or charging and cannot be effectively controlled or cooled. This may result in severe safety incidents such as overheating, fire, or explosion.
Why Do EV Batteries Ignite So Quickly?
EVs commonly use lithium-based batteries, which are electrochemical in nature. Under extreme conditions, electrode short-circuiting can occur, leading to intense chemical reactions. A damaged battery generates heat and may ignite. Moreover, the presence of flammable materials within the vehicle—such as seats—can accelerate the spread of fire.
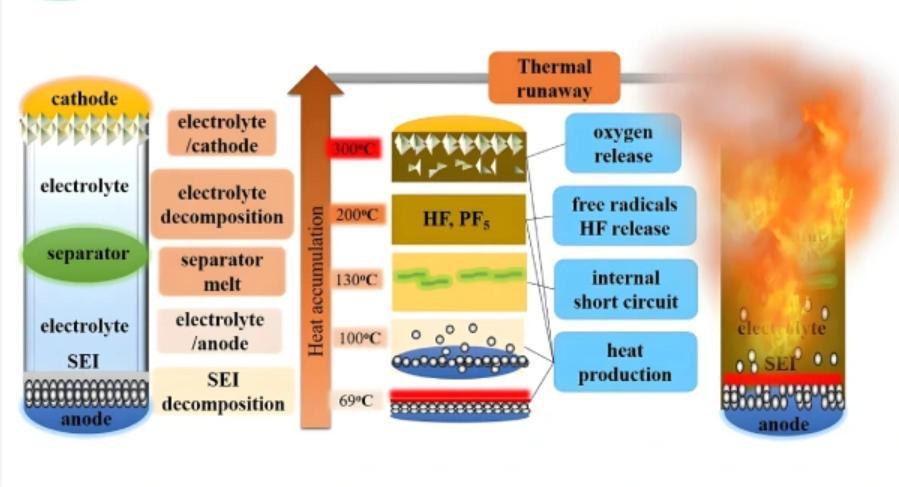
During thermal runaway in lithium-ion batteries, multiple reactions take place, including:
- Decomposition of the solid electrolyte interface (SEI) film
- Reactions between the active material (cathode or anode) and the electrolyte
- Electrolyte decomposition
- Reaction between the anode active material and the binder
Under high temperatures, the capacity of lithium-ion batteries decreases significantly, while the internal resistance of the electrodes increases—more notably at the anode.
When temperatures exceed 80°C, thermal decomposition begins. Between 80–120°C, the SEI film decomposes, exposing the anode active material. This exposed lithium metal can then react with the electrolyte.
As temperatures continue to rise, the porous separator—often made of polyethylene (PE) or polypropylene (PP)—begins to melt. The melting points are around 130°C and 170°C, respectively. This melting can initially block current flow and provide some self-protection. However, around 190°C, the separator breaks down entirely, leading to an internal short circuit.
Excessive current induces rapid temperature rise, triggering decomposition of the cathode and electrolyte. The cathode decomposition releases substantial heat, a key contributor to thermal runaway.
Additionally, the decomposition of cathode materials releases oxygen. The rapid accumulation of gas increases internal pressure. If this exceeds the pressure limit of the battery safety valve, venting or jetting occurs, posing serious safety risks.
Thermal runaway typically progresses through three stages: initiation, occurrence, and propagation. Common causes include:
- Overheating
- Overcharging
- Internal short circuits
- Physical damage (e.g., from collisions)
Triggers of Thermal Runaway:
1. Mechanical Abuse: Damage from collisions, crushing, or penetration leading to physical deformation and internal short circuits.
2. Electrical Abuse: External short circuits, overcurrent, or overcharging that exceed the battery’s tolerance, generating excessive heat.
3. Thermal Abuse: Exposure to high temperatures beyond the battery’s safe limit, often due to cooling system failure or heat from adjacent cells.
These triggers are often interrelated. Mechanical abuse can lead to electrical abuse, which generates heat leading to thermal abuse, ultimately initiating a chain reaction toward thermal runaway.
Although LiFePO4 batteries are known for their thermal stability, they can still experience thermal runaway due to:
1. Internal Short Circuits: Caused by manufacturing defects, usage damage, or electrolyte leakage.
2. Overcharging or Over-discharging: Leading to internal temperature rise.
3. External Factors: High ambient temperature, physical crushing, or puncture.
1. Performance Degradation: Reduced capacity, charging efficiency, and lifespan.
2. Safety Hazards: Risk of fire or explosion, endangering lives and property.
3. Vehicle Failure: Loss of power or inability to operate, affecting EV functionality.
1. Battery Balancing: Ensure uniform charge/discharge across all cells.
2. Cooling System Optimization: Use liquid cooling or heat pipes to improve heat dissipation.
3. Thermal Management System: Implement real-time temperature monitoring and control.
4. Safety Inspections: Use X-ray or ultrasonic testing to detect internal defects.
5. Quality Control: Improve manufacturing consistency and reliability.
6. Proper Usage and Maintenance: Avoid improper charging practices.
7. Emergency Protocols: Establish response plans for thermal runaway incidents.
8. Battery Recycling: Develop systems for safe disposal and recycling of used batteries.
9. Research & Development: Invest in safer battery materials and designs.
10. Policy and Regulation: Enforce strict industry standards and safety regulations.
Thermal runaway in LiFePO4 batteries is a complex issue requiring multidisciplinary solutions. Through effective management, cooling, monitoring, and regulatory measures, the risks can be mitigated. Continued research and development are essential to advancing LiFePO4 battery technology and supporting the safe growth of the electric vehicle industry.
Can LiFePO4 batteries go into thermal runaway?
Yes, though they are more stable than other lithium-ion chemistries, they can still experience thermal runaway under extreme conditions.
What is the thermal runaway temperature of LFP batteries?
Typically above 250°C, higher than most other lithium-ion batteries.
What gases are released during LFP thermal runaway?
LFP batteries produce little to no hazardous gases, making them safer in case of failure.
Can all lithium batteries undergo thermal runaway?
Yes, all lithium-based batteries can experience thermal runaway if subjected to abuse conditions such as overheating, overcharging, or physical damage.
Next:Sungrow Unveils Groundbreaking Inverters and Energy Storage Systems at RE+ 2025
Previous:EVE Energy Unveils Large Cylindrical Battery and Battery Passport at IAA Mobility 2025